Abstract
Introduction: High-dose methotrexate (HD-MTX) remains a backbone therapy in a number of malignancies and is defined as a dose of greater than 500 mg/m2. HD-MTX requires supportive measures to minimize toxicity, particularly nephrotoxicity. MTX levels are followed to ensure adequate clearance of drug. Historically, patients were discharged with a MTX level of ≤0.05 umol/L. However, many single-center studies have shown that liberalizing the discharge level to <0.1 umol/L is safe and allows earlier hospital discharge. At NYU Langone Health (NYULH), many patients with stable renal function are discharged with a level of >0.1 umol/L, with supportive medications including oral leucovorin with or without sodium bicarbonate. The aim of our study is to confirm the safety of discharging patients with a MTX level of >0.1 umol/L.
Methods/Results: This was a single center, retrospective, institutional-review board approved study of all adult patients who received HD-MTX in the inpatient setting, between January 1, 2017 and February 20, 2021. The primary endpoint was the incidence of renal injury post discharge from the HD-MTX admission. Outcomes were analyzed among three MTX level categories at discharge (>0.2 umol/L, 0.1-0.2 umol/L, <0.1 umol/L). If patients received more than one cycle of HD-MTX, each cycle was reported as an individual event. Baseline characteristics were reported based on the first infusion of each unique individual. Baseline renal insufficiency was defined as a creatinine clearance (CrCl) of less than 30 mL/min. Renal injury was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE, version 5.0). The first outpatient serum creatinine (SCr) documented in the patient chart post HD-MTX hospitalization was utilized to analyze renal injury.
The study included 108 patients who received a total of 336 HD-MTX infusions. The primary indication for HD-MTX was central nervous system prophylaxis for diffuse large b-cell lymphoma or acute lymphoblastic leukemia. The most common HD-MTX doses were between 1,001-3,500 mg/m2 compared to 500-1,000mg/m2 and >3,500mg/m2 (p=0.0006). Four patients (4%) had baseline renal insufficiency. Of the 336 infusions, 49 (15%) infusions were categorized into MTX discharge level >0.2 umol/L, 145 (43%) into MTX 0.1-0.2 umol/L, and 142 (42%) into MTX <0.1 umol/L. Within the discharge category >0.2 umol/L, the median level was 0.28 umol/L (IQR, 0.22 - 0.37). The highest level was 1.04 umol/L at discharge. Patients were more likely to be discharged with oral leucovorin if they were discharged with a MTX level >0.1 umol/L (p<0.001). Similarly, patients were more frequently prescribed oral sodium bicarbonate if discharged with a MTX level >0.2 umol/L (p<0.001).
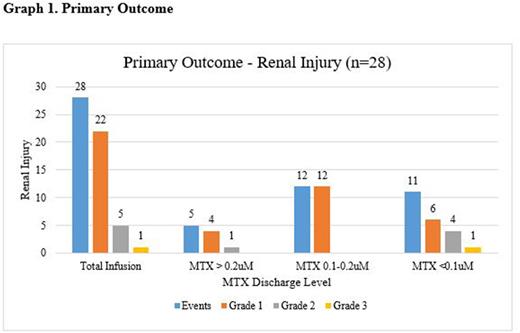
There were 28 (8%) renal injury events, with no significant difference in renal injury occurring among the three MTX discharge level ranges. The renal injury events were primarily Grade 1. The median LOS in our study was statistically longer in the MTX <0.1 umol/L category (4 days, IQR 3-12) compared to the MTX 0.1-0.2 umol/L and >0.2 umol/L categories, 3 days (IQR 3-4) and 3.4 days, (IQR 3-4), respectively (p=0.023). There were 14 readmissions post HD-MTX hospitalization discharge. Two patients were admitted on two separate encounters after HD-MTX administration, leaving 12 unique patients. The primary reason for readmission was for myelosuppression, although all of these patients received other cytotoxic chemotherapy as part of the hyper-CVAD regimen. Two readmissions were after a MTX discharge level of >0.2 umol/L and 6 readmissions for both MTX discharge level of 0.1- 0.2 umol/L and <0.1 umol/L.
Conclusion: Our study demonstrates that it is safe to discharge patients with a MTX level of >0.1 umol/L, with no difference in renal injury, and supporting shortened length of stay. Our data also suggest that it is safe to discharge a patient with a MTX level up to 0.4 umol/L, as our upper 75th quartile range was 0.37 umol/L, acknowledging supportive medications are prescribed. Limitations of this study include the retrospective nature of the study and heterogenous population. The length of stay data are confounded by other factors as well including multiday chemotherapy regimens, infections, and newly diagnosed malignancies. Our plan is to create an institutional protocol to outline the supportive care to support an early discharge of patients receiving HD-MTX.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal